Read also:
Dietary supplements are widely available in the United States and Canada. Still, they can also cause serious harm if made incorrectly or contain contaminated ingredients. Standard Process Laboratories (SPL) is a dietary supplement manufacturer in Minnesota. The company sells supplements under the brand names Daily Balance and SP Therapeutics. This company manufactures dietary supplements to treat various conditions, including autism, digestive disorders, cancer, heart disease, and diabetes.
December 8, 2018 – Liable for Fraud and Deceptive Marketing
On December 8, 2018, a federal jury found Standard Process Laboratories liable for fraud and deceptive marketing. The jury awarded $5.5 million to the plaintiff, who sued after purchasing one of SPL’s products, “Cholestin.”
The plaintiff claimed that Cholestin did not work as advertised: it didn’t reduce blood cholesterol levels or improve heart health. He also alleged that SPL falsely claimed its other products’ health benefits, including Calcium D-Glucarate and DIM (diindolylmethane).
The lawsuit was filed by a former employee of Standard Process Laboratories (SPL), a Utah-based dietary supplement manufacturer. It was filed against SPL and its owner, Robert Scott Johnson, in the United States District Court for the District of Utah on February 19, 2019.
The complaint alleges that SPL engaged in unfair labor practices and tortiously interfered with its employees’ rights under state law by requiring them to sign an arbitration agreement as part of their employment contracts that prohibited them from filing lawsuits against employers or taking other legal action against employers if they were terminated or laid off from work.
The Management

Standard Process Laboratories, Inc., is a nutritional supplement company in Palmyra, Wisconsin. It was founded by Royal S. Lee (1895–1967), a nonpracticing dentist who was interested in vitamins and nutrition. The company began as the Vitamin Products Company, which sold food supplements, and later became the Lee Foundation for Nutritional Research.
The company was managed by Dr. William J. Leininger in 1970 as a nutritional supplement company focused on giving people high-quality products at affordable prices. His son Mark took over as CEO after his father’s death in 1995 and had been running things ever since with his wife, Sharon Leininger-Baumann (Chief Financial Officer).
The company has changed hands several times since its inception and is now owned by a family trust. However, it still operates as Standard Process, Inc. and sells supplements, herbal, and veterinary products.
Glassdoor estimates the company’s annual gross sales to be between $100 and $500 million. Its catalogs have described the ingredients of its products as vitamins, minerals, protein isolates, glandular extracts, organ extracts, dried tissues, plant material, and plant extracts, singly or in various combinations.
SPL markets its products through direct marketing channels such as chiropractors’ offices or online sales sites. The company also sells products through retail stores and healthcare providers.
Is Standard Process a pyramid scheme?
There is no evidence to suggest that Standard Process, a company that produces dietary supplements and other health products, is a pyramid scheme. Pyramid schemes are illegal and are characterized by a focus on recruitment and the promise of large financial rewards for those who recruit others into the scheme. Standard Process does not engage in recruitment-based compensation, nor does it rely on new recruits to generate income. Rather, the company sells its products directly to consumers and through healthcare professionals, and it compensates its independent distributors based on product sales. While some individuals may choose to build a business by selling Standard Process products and recruiting others to do the same, this is not the primary focus of the company’s business model, and there is no evidence to suggest that it operates as a pyramid scheme.
Dietary supplements can cause serious harm if made incorrectly or contain contaminated ingredients.
The manufacturing of dietary supplements is a highly regulated process. Therefore, it’s important to ensure that the quality control measures are in place and followed by all parties involved.
- Test for contaminants: One-way manufacturers can ensure their products’ purity through testing them. This can be done through physical and chemical analysis methods, such as infrared spectroscopy or chromatography.
- Test for purity: In addition to testing for contaminants, it’s also important that you know exactly how much of each ingredient is present in your supplement before selling it on the market or giving it away free of charge (such as with clinical trials).
Product Lines and Under Standard Process
Standard Process is a supplement company that offers a wide range of products, including whole food supplements, herbal supplements, and other dietary supplements. The company has been in business for over 90 years and is known for its commitment to producing high-quality supplements made from whole foods and natural ingredients. With a commitment to using whole foods and natural ingredients, this company has established a reputation for quality and effectiveness in the supplement industry. Under the Standard Process brand, there are several product lines, including:
Under the Standard Process brand, there are several product lines, including:
- Standard Process: This product line includes whole food supplements, herbal supplements, and other dietary supplements.
- MediHerb: This line of products focuses on herbal supplements that are designed to support specific health conditions.
- Standard Process Veterinary Formulas: This line of products includes supplements and other products designed for use in animals.
- Standard Process Equine Formulas: This line of products includes supplements and other products designed for use in horses.
- Standard Process Labs: This line of products includes clinical laboratory services for healthcare professionals.
- SP Complete: This is a meal replacement shake mix that is designed to provide essential nutrients and support overall health.
- SP Cleanse: This is a detoxification program that is designed to support the body’s natural cleansing processes.
- SP Complete Dairy Free: This is a dairy-free version of the SP Complete meal replacement shake mix.
- SP Green Food: This is a supplement that contains a blend of green vegetables and other plant-based ingredients.
- SP Immune Support: This is a supplement that is designed to support immune system function.
- SP Metabolic Support: This is a supplement that is designed to support healthy metabolism and energy levels.
- SP Men’s Health: This is a supplement that is designed to support men’s health and vitality.
- SP Women’s Health: This is a supplement that is designed to support women’s health and vitality.
- SP Stress Management: This is a supplement that is designed to support healthy stress levels and relaxation.
- SP Systemic Formulas: This line of products includes supplements and other products designed to support various aspects of health and wellness.
Standard Process Supplement Reviews
One of the unique aspects of Standard Process is their focus on using whole foods and natural ingredients in their products. In addition to its main product line, Standard Process offers several sub-brands, each with a specific focus and purpose. Let’s take a closer look at some of the top brands under Standard Process and explore what sets them apart from other supplements on the market.
Antronex is a dietary supplement manufactured by Standard Process designed to support liver and gallbladder health. It contains various natural ingredients, including bovine liver fat extract and vitamin E, which are believed to promote healthy liver function and aid in the digestion of fats.
Bio-Dent is a dietary supplement designed to support healthy bones and teeth. It contains various natural ingredients, including bovine bone, calcium lactate, and magnesium citrate, which promote healthy bone growth and mineralization.
Calcifood is a dietary supplement designed to support healthy bone growth and development. It contains various natural ingredients, including bovine bone, calcium lactate, and magnesium citrate, which promote healthy bone growth and mineralization.
Cardio-Plus is a dietary supplement that is designed to support cardiovascular health. It contains various natural ingredients, including bovine heart extract, hawthorn berry, and vitamin C, which promote healthy blood flow and circulation.
Cardio-Plus is a dietary supplement designed to support cardiovascular health. It contains a blend of nutrients, including vitamins, minerals, and bovine heart extract, which is rich in nutrients that are beneficial for heart health. Cardio-Plus is formulated to support healthy blood flow and circulation, as well as to provide antioxidant support.
Catalyn is a whole-food supplement that contains a blend of vitamins, minerals, and other nutrients derived from whole foods. It is designed to support overall health and wellness by providing the body with a broad range of nutrients that are essential for optimal health. Catalyn is made with high-quality ingredients and is designed to be easily absorbed and utilized by the body.
Chezyn is a dietary supplement designed to support healthy digestion. It contains a blend of digestive enzymes, bile salts, and other ingredients that are designed to promote healthy digestion and absorption of nutrients. Chezyn is formulated to support healthy bile flow and to aid in the breakdown of fats, proteins, and carbohydrates.
Cholacol is a dietary supplement designed to support healthy cholesterol levels. It contains a blend of bile salts and bovine liver extract, which are believed to promote healthy cholesterol metabolism and excretion. Cholacol is designed to support healthy liver function and to promote healthy cholesterol levels.
Cyruta is a dietary supplement designed to support healthy circulation. It contains a blend of nutrients, including vitamin C and extracts of buckwheat and sweet orange, which are believed to promote healthy blood flow and circulation. Cyruta is designed to support the health of the circulatory system and to promote overall cardiovascular health.
DermaCo is a dietary supplement designed to promote healthy skin. It contains a combination of vitamins, minerals, and herbal extracts that support skin health and reduce the signs of aging.
Diaplex is a dietary supplement designed to support healthy blood sugar levels. It contains a blend of vitamins, minerals, and herbal extracts that help regulate blood sugar levels and improve insulin sensitivity.
E-Poise is a dietary supplement designed to support healthy adrenal function. It contains a blend of vitamins, minerals, and herbal extracts that help regulate the stress response and promote overall adrenal health.
E-Z Mg is a dietary supplement designed to support healthy magnesium levels in the body. Magnesium is an essential mineral that is required for many bodily functions, including muscle and nerve function, energy metabolism, and bone health.
Emphaplex is a dietary supplement designed to support healthy lung function. It contains a blend of vitamins, minerals, and herbal extracts that help reduce inflammation in the lungs and support respiratory health.
Gastrex is a line of products that focuses on digestive health. Gastrex supplements contain a variety of natural ingredients, including slippery elm bark, okra, and bentonite clay, which are believed to support healthy digestive function and alleviate symptoms such as acid reflux, bloating, and gas.
Immuplex is a line of supplements designed to support immune system function. These supplements contain a blend of vitamins, minerals, and other natural ingredients, such as bovine thymus, which is believed to help support the health and function of the thymus gland, a key component of the immune system.
Ligaplex is a line of supplements designed to support musculoskeletal health. These supplements contain a blend of vitamins, minerals, and other natural ingredients, such as bovine cartilage and bromelain, which are believed to support joint health and flexibility, as well as healthy ligaments and tendons.
Livaplex is a line of supplements designed to support liver function. These supplements contain a blend of vitamins, minerals, and other natural ingredients, such as milk thistle and dandelion root, which are believed to support healthy liver function and detoxification.
MediHerb: MediHerb is a brand under Standard Process that specializes in herbal supplements designed to support specific health conditions. Their products are made with high-quality herbs and are formulated based on extensive research and clinical trials. They offer a wide range of products, including digestive support, immune system support, and cardiovascular health supplements.
Min-Tran: Min-Tran is a supplement under Standard Process that is formulated to support a healthy nervous system. It contains a unique blend of minerals, including calcium, magnesium, and iodine, which work together to support healthy nerve function and reduce stress and anxiety.
Okra Pepsin E3: Okra Pepsin E3 is a digestive supplement under Standard Process that is formulated to support healthy digestion. It contains a blend of natural ingredients, including okra, pepsin, and calcium, which work together to support the breakdown of food and the absorption of nutrients.
Organically Bound Minerals (OBM): Organically Bound Minerals (OBM) is a supplement under Standard Process that is formulated to support healthy mineral balance in the body. It contains a blend of minerals that are bound to organic molecules, which makes them more easily absorbed and utilized by the body.
Paraplex: Paraplex is a supplement under Standard Process that is formulated to support healthy adrenal function. It contains a blend of vitamins and minerals, including vitamin C, vitamin B6, and magnesium, which work together to support the body’s natural stress response and promote overall wellness.
ProSynbiotic: ProSynbiotic is a probiotic supplement under Standard Process that is formulated to support healthy digestion and immune function. It contains a blend of probiotics and prebiotics, which work together to support the growth of healthy gut bacteria and promote overall digestive health.
Renafood: This supplement is designed to support healthy kidney function and includes a variety of whole food ingredients, such as bovine kidney, as well as other herbs and nutrients that are believed to promote kidney health.
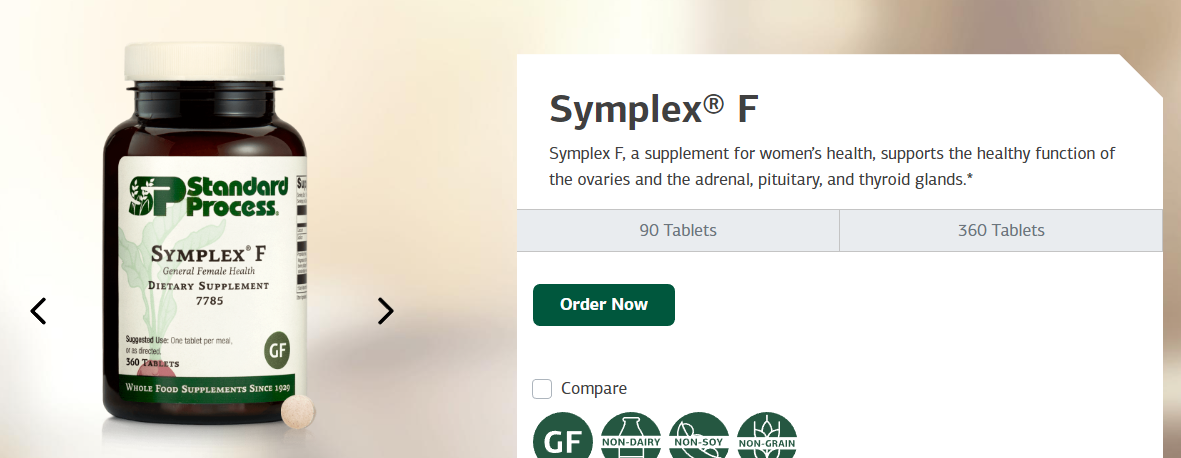
Symplex F: Symplex F is a dietary supplement that is designed to support healthy function of the female reproductive system. It contains a blend of glandular extracts, including bovine ovary and uterus, as well as other nutrients that are important for female health. Symplex F is intended to help balance hormone levels and promote healthy menstruation.
Congaplex is a dietary supplement designed to support the immune system and promote overall health. It contains a variety of natural ingredients, including bovine thymus, spleen, and liver extracts, as well as several herbs that are believed to have immune-boosting properties. Congaplex is intended to be used as a preventative measure during cold and flu season, as well as to support recovery from illness. While it is generally considered safe and well-tolerated, some users may experience side effects such as stomach upset or allergic reactions. It is important to follow the recommended dosage and usage instructions carefully, and to consult with a healthcare professional before taking Congaplex or any other dietary supplement.
Ferrofood is a dietary supplement formulated to support healthy iron levels in the body. It contains natural sources of iron such as bovine liver, spleen, and bone, as well as vitamin C to enhance iron absorption and utilization. While it is generally considered safe and effective, some users may experience side effects such as nausea, constipation, and stomach cramps. Additionally, Ferrofood may interact with certain medications and cause iron overload in rare cases. It is important to consult a healthcare professional before taking Ferrofood and to follow the recommended dosage and usage instructions carefully.
Thymex: Thymex is a dietary supplement that is designed to support the function of the thymus gland. The thymus gland plays an important role in the immune system, and Thymex contains a blend of glandular extracts and other nutrients that are believed to help support thymus function. Thymex is intended to promote immune system health and function.
Trace Minerals-B12: Trace Minerals-B12 is a dietary supplement that is designed to provide the body with essential trace minerals, including zinc, copper, chromium, and selenium, as well as vitamin B12. These nutrients are important for a wide range of bodily functions, including immune system health, energy production, and nervous system function. Trace Minerals-B12 is intended to promote overall health and well-being.
Tuna Omega-3 Oil: Tuna Omega-3 Oil is a dietary supplement that is designed to provide the body with omega-3 fatty acids, which are important for heart health, brain function, and overall health and well-being. Tuna Omega-3 Oil is derived from wild-caught tuna and is a natural source of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), two types of omega-3 fatty acids that are essential for health.
Zypan: Zypan is a dietary supplement that is designed to support healthy
digestion and the breakdown of food in the stomach. It contains a blend of betaine hydrochloride, pepsin, and pancreatic enzymes, which are important for the digestion of protein, carbohydrates, and fats. Zypan is intended to promote healthy digestion and nutrient absorption.
Overall, the Standard Process product line includes a variety of whole food supplements, herbal supplements, and other dietary supplements designed to support overall health and well-being. While some products may not be suitable for everyone, the company’s commitment to using high-quality ingredients and sustainable farming practices has made it a trusted source of natural health products for over 90 years.
Conclusion
It’s important to remember that dietary supplements can cause serious harm if made incorrectly or contain contaminated ingredients. It’s also important for consumers to know what type of product they’re buying and whether it has been tested by the Food and Drug Administration (FDA).